ENFIT Innovative Nutritional Delivery
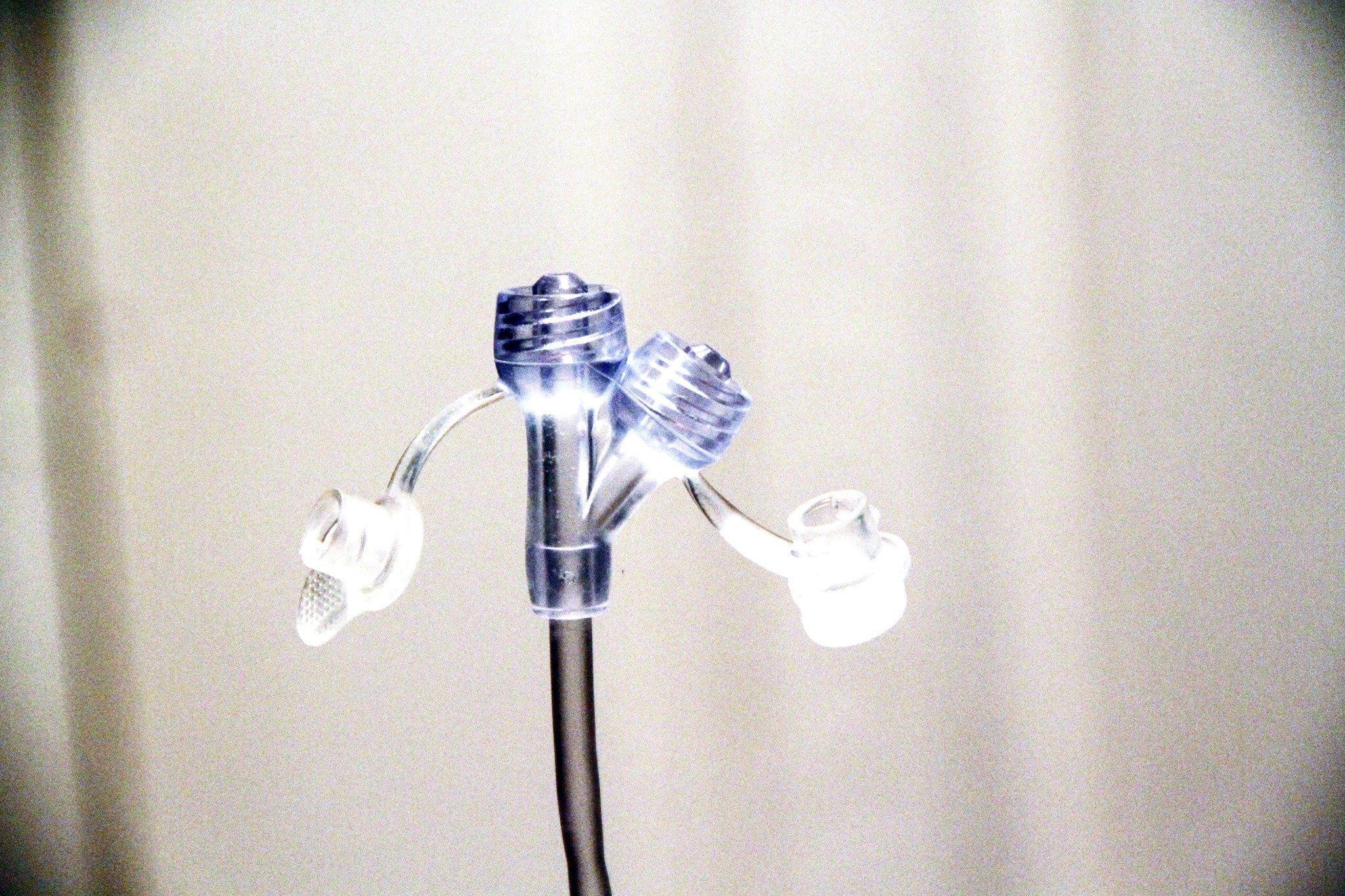
What is ENFIT?
ENFit is a global innovative change in nutritional delivery products, with a mission to make all enteral (tube feeding) devices specific to tube feeding. In line with this change, every extension set, syringe, long tube/PEG, and NG-tube will be designed with a specific ENFit end so that you can only use products designed for enteral/tube feeding access. Button style G-tubes will not change at all — only the extension sets that connect to them.
Recently, the International Standards Organization created ISO CD 80369-3 to help reduce the frequency of medical tubing misconnections. ENFIT features a unique design to reduce the risk of enteral feeding tube misconnections and improve patient safety.
Why The Change?
Way back in 2006, many in the enteral community expressed concern about the ability of medical devices to connect across systems. For example, so that syringes used for tube feeding would also fit into IV systems of TPN patients. Cases of misconnections are majorly under-reported and many may be assessed as medication errors. Several people have die from misconnections and many more are put at serious risk. In our pediatric community, there is also a very serious risk of disconnection. Current systems, particularly med ports, open easily, making it possible for our children to miss feeds for hours, particularly overnight. This is an inconvenience for most, but a serious problem for children who have issues maintaining their blood sugars.
It took several years to get all manufacturers of enteral products around the world together to move to a standard connection. A trade group was formed called GEDSA to work through the transition. The roll out is worldwide with staggered timing. There is a lengthy transition period during which both the old and the new enteral products will be available.
Where We Are Now and Moving Forward
ENFit versions are available for all product lines in the United States. However, some durable medical equipment providers have not transitioned over. We estimate about 2/3 of families are receiving ENFit versions and 1/3 are receiving the older versions as of 2020. It will take time for supply companies to work through their stock of supplies with the old connectors before they start mass distribution of the new ENFit supplies.
Quick facts!
- AMT has released a series of adaptors so that you can continue to use your current supplies and stockpiles
- It will take time for supply companies to work through their stock of supplies with the old connectors before they start mass distribution of the new ENFit supplies.
- Low-dose ENFit syringes have been redesigned to prevent the medication errors that were a concern with preliminary designs. There are also adapter caps to ensure accurate dosing.
- There is ongoing testing on various commercial formulas and blenderized food products to ensure that tube feeders’ dietary choices will not be impacted by the move to ENFit.
- Multiple companies have created special cleaning brushes to allow for thorough cleaning of all ENFit connectors.